Hydrogen exists all around us. Through rapid advances in means for “observing” hydrogen, we are gradually uncovering the various roles that hydrogen plays in materials.
 Where is the hydrogen?
Where is the hydrogen?
Chronicle
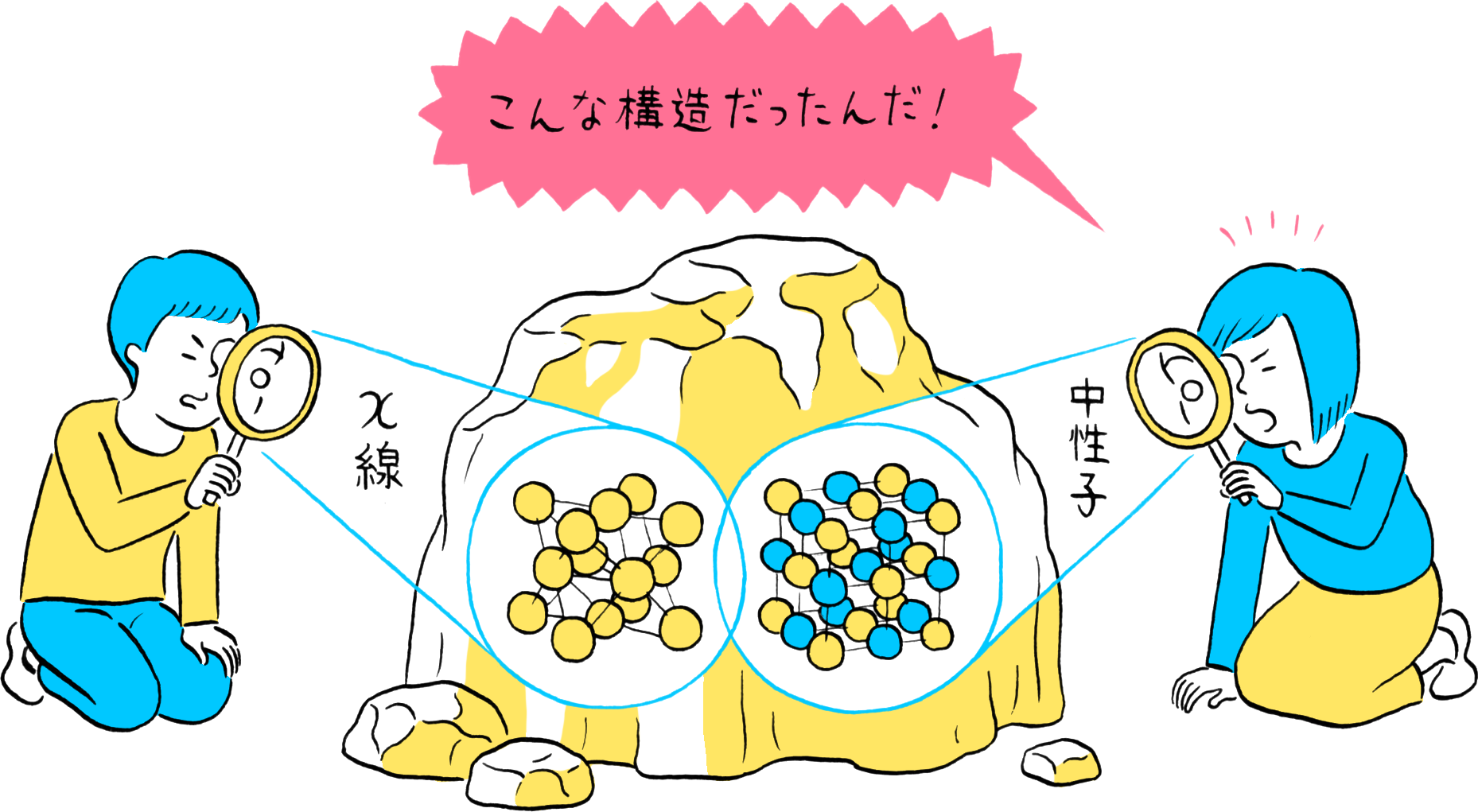
Hydrogen is present everywhere. The sun is made up of 85% (atomic ratio) hydrogen, while the human body and seawater comprise about 60% (atomic ratio) hydrogen. Naturally, most of the matter around us contains hydrogen. However, the location of hydrogen and its role in matter was not well known until modern times because we had no means of “observing” hydrogen.
Experiments to study crystal structures began in the 1910s, initially with the use of X-rays. Some twenty years later, particles called neutrons that constitute the nuclei of atoms were discovered, and in the 1940s experiments using neutrons were initiated to study atomic structures. Thereafter, these two “observing means” utilizing X-rays and neutrons were developed together, but for a long time it was thought that hydrogen could not be seen using X-rays and could only be observed with neutrons. The reason for this belief lies in their different viewing mechanisms.
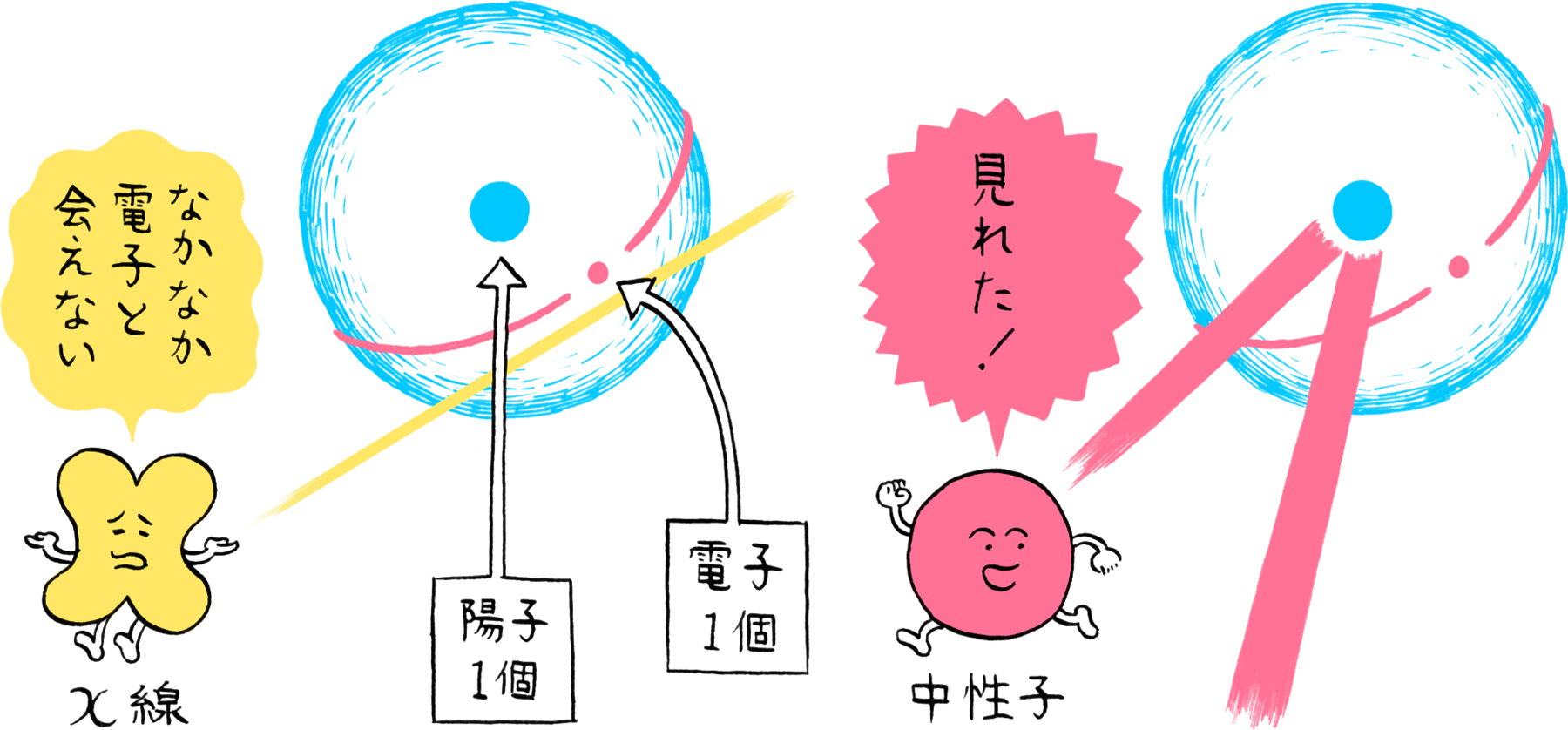
When irradiated on crystal, X-rays are scattered by the atoms in the crystal. Since X-rays possess wave properties, they interfere with each other and are diffracted in specific directions. This pattern of diffraction reflects the arrangement of atoms, making it possible to identify the atomic arrangement based on the diffraction pattern. In fact, the X-rays are scattered by the electrons orbiting the nuclei. Thus, X-rays are more likely to be scattered and are easier to observe for atoms that have a larger number of electrons. In other words, atoms having a larger number of electrons are more visible. Since hydrogen atoms have only a single electron, their visibility with X-rays is less than one-fortieth that of carbon atoms, which possess six electrons.
 Using neutrons to see hydrogen
Using neutrons to see hydrogen
Since neutrons also possess wave properties, the arrangement of atoms can be determined by measuring neutron diffraction. Neutrons also possess no charge. Therefore, they have no interaction with electrons and can slip through the electron cloud formed by the orbiting electrons to be scattered by the nuclei. Consequently, their visibility is unaffected by the number of electrons possessed by an atom, enabling hydrogen to be observed the same as other elements. However, to utilize neutrons as an “observing” means, a large number of neutrons must be produced from atomic nuclei. Consequently, experiments utilizing neutrons require large-scale facilities including reactors and accelerators for producing millions of neutrons each second, and advanced technologies for irradiating a concentrated beam of neutrons onto the sample.
NOVA, an Advanced Experimental Instrument for Observing Hydrogen
Beginning in the 1960s, Japan began conducting materials science research with neutrons using the JRR-2 reactor at the Japan Atomic Energy Research Institute, Tokai Research Establishment (present-day Nuclear Science Research Institute of the Japan Atomic Energy Agency). In the latter half of the 1970s, Japan began conducting neutron scattering experiments using an electron accelerator at Tohoku University. The technology for these experiments was developed at the National Laboratory for High Energy Physics (present-day High Energy Accelerator Research Organization; KEK) and has been passed on to the Materials and Life Science Experimental Facility (MLF) at the Japan Proton Accelerator Research Complex (J-PARC) completed in 2008.
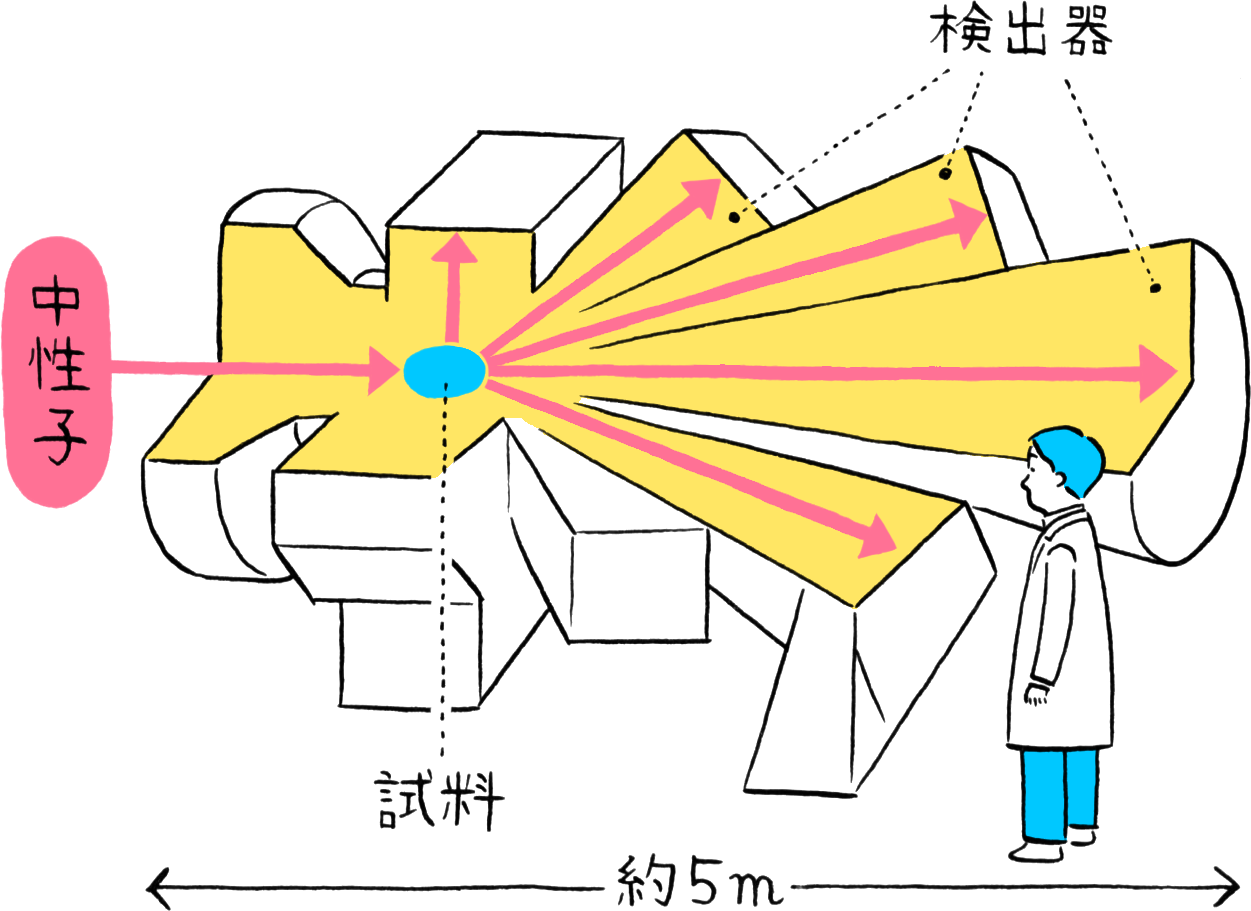 Experimental instrument NOVA using neutrons for observation
Experimental instrument NOVA using neutrons for observation
MLF has numerous instruments for observing atomic arrangements (structures) using neutrons, one of which is the High Intensity Total Diffractometer (NOVA). NOVA was designed with the intention of observing hydrogen in matter. It is a cutting-edge experimental instrument capable of conducting high-sensitivity evaluations in the world’s shortest time for advanced materials that can only be synthesized in small quantities. Further, while common instruments used in diffraction experiments can only evaluate crystal structures with ordered arrangements of atoms, NOVA supports total scattering experiments for evaluating disordered structures such as liquids. The atomic structures that are the source of a material’s properties can be clarified by varying the temperature and pressure of the sample.
However, a material’s properties and functions are also greatly influenced by the states of electrons, not just the atomic arrangement. Accordingly, X-rays must be used to study electron states since electrons cannot be observed with neutrons. X-rays also have other characteristics not possessed by neutrons, such as the ability to observe the electron states of specific elements and to perform observations using a beam diameter of several hundred nanometers.
“Neutrons are the first step. Once the atomic structure is known, it is preferable to use X-ray beams as a complement to neutrons.”
Dr. Toshiya Otomo, who has developed and experimented with NOVA, emphasizes this coordination with X-ray beams.
 An instrument for conducting neutron diffraction experiments with a sample preserved in a hydrogen-gas atmosphere
An instrument for conducting neutron diffraction experiments with a sample preserved in a hydrogen-gas atmosphere
 NOVA has numerous neutron detectors arranged three-dimensionally to capture neutrons scattered in all directions by the (partial) sample
NOVA has numerous neutron detectors arranged three-dimensionally to capture neutrons scattered in all directions by the (partial) sample
The World of Materials Expanded by Neutrons
In order to realize a hydrogen society, the improved performance of hydrogen storage materials is a great need. As basic research for this goal, Dr. Otomo is conducting neutron experiments aimed at elucidating the structures of metal hydrides and the storage and release mechanisms of hydrogen.
Neutrons are not only useful in observing hydrogen, but are also excellent for observing lithium and other light elements. Research being conducted in the Element Strategy Initiative includes analyses of such elements aimed at improving the performance of lithium batteries and developing substitute materials. Recently this research has enabled us to observe changes in the positions of lithium as well as its movement during charging and discharging.
“These results are only possible at J-PARC due to the need for a large quantity of neutrons in the experiments,” Dr. Otomo says with pride.
 Dr. Otomo (Head of the Neutron Science Division of the Institute of Materials Structure Science, KEK) conducts neutron research at J-PARC
Dr. Otomo (Head of the Neutron Science Division of the Institute of Materials Structure Science, KEK) conducts neutron research at J-PARC
Neutrons have another significant property: spin. With spin, neutrons behave like magnets, enabling us to learn the magnetic structures of the materials. That is, with the ability to view hydrogen and magnetism simultaneously, we can utilize these properties to study the effects of hydrogen on magnetism. These properties are also useful for investigating magnetic materials that can replace neodymium magnets and for research on superconductors.
Neutrons are utilized in the field of life sciences, as well. Life forms are teeming with hydrogen, and researchers believe that the positions of hydrogen, particularly in amino acids and proteins involved in functional expressions, are of utmost performance. Here, we also perform the specialized work of determining frameworks constructed of carbon, oxygen, and nitrogen in crystal structure analyses using X-rays and use neutrons to identify the positions of hydrogen, which are difficult to see with X-rays.
Neutrons are further capable of penetrating materials to capture internal images, much like X-ray photographs. Although X-rays easily penetrate plastics and other materials formed of light elements, they do not readily penetrate iron or other materials formed of heavy elements. Therefore, neutrons are used in nondestructive inspections for detecting internal defects of large and heavy machinery, such as automobile engines and structures, which X-rays have difficulty penetrating. Applications for neutrons are expected to continue to expand in industry and society. Even now, industrial utilization accounts for 30% of the experiments conducted at MLF.
 Experiment Hall at MLF
Experiment Hall at MLF
Additional Challenges
“Advances in computational science have enabled us to predict positions in metal hydrides that hydrogen will occupy. However, such predictions remain difficult because the sites that hydrogen atoms occupy change when the distance between metal atoms is shrunk through applied pressure, for example. Since too little is known regarding interactions and bonding between metal and hydrogen, especially under high pressure, there is still much need to conduct direct observations with neutrons.”
“Assiduous efforts to conduct experiments again and again are needed to accurately determine positions of hydrogen in materials.”
Dr. Otomo told us the following about the current state of neutron research.
“The current goal for neutron diffraction experiments is to improve spatial and time resolution. For this purpose, we will need to develop technologies for producing more neutrons and for focusing a beam of the neutrons on a sample.”
As individual researchers, they are attempting to look further into the future.
“I would like to be able to distinguish the differences between light hydrogen and heavy hydrogen in observations. Through advances in observation techniques using X-ray beams, it is now possible to observe hydrogen with X-rays. I would like to gain mastery of observation technology to its extreme in order to determine what it actually means to observe hydrogen with neutrons.”
We asked a young researcher who works with Dr. Otomo about his goals. Associate Professor Kazutaka Ikeda is focused on basic research for generating hydrogen energy, which will be needed in a hydrogen society. His response was, “I think it would be interesting if we could increase the intensity of neutrons by an order of magnitude over that at J-PARC, for example. That would enable us to take a snapshot of a fuel cell’s interior at the instant hydrogen energy is generated by chemical reaction.”
 One of the young neutron researchers, Kazutaka Ikeda (Associate Professor in the Neutron Science Division of the Institute of Materials Structure Science, KEK)
One of the young neutron researchers, Kazutaka Ikeda (Associate Professor in the Neutron Science Division of the Institute of Materials Structure Science, KEK)
Assistant Professor Takashi Honda remarked that, “It is interesting to have the capability to experiment under ultrahigh pressure. When confined under an ultrahigh pressure, hydrogen sulfide becomes superconductive at the considerably high temperature of 200K. Such a high superconductive temperature is thought to be the result of interactions between hydrogen and superconductivity, and the arrangement of hydrogen atoms is said to hold the key. In addition to superconductivity, numerous other changes in properties attributed to hydrogen occur under ultrahigh pressures. Therefore, I believe this is an age in which neutron experiments under ultrahigh pressures are indispensable.”
It appears that the pursuit of “observing hydrogen” has only just begun.
 One of the young neutron researchers, Takashi Honda (Assistant Professor in the Neutron Science Division of the Institute of Materials Structure Science, KEK)
One of the young neutron researchers, Takashi Honda (Assistant Professor in the Neutron Science Division of the Institute of Materials Structure Science, KEK)
Illustrations by Takumi Shirai, photographs by Michito Ishikawa

